Abstract
INTRODUCTION: Allogeneic hematopoietic stem cell transplantation (allo-HSCT) holds the potential for cure of numerous blood diseases and provides the best chance for durable control of acute myeloid leukemia (AML). Successful allo-HSCT requires that host hematopoietic stem cells (HSCs) are depleted to make bone marrow (BM) space for the incoming donor HSCs, and that host T and NK cell mediated immunity is suppressed to prevent donor HSC rejection. To meet these requirements, conditioning regimens comprised of chemotherapy and/or irradiation are used, which carry risks of severe toxicities that may prevent patients from undergoing this potentially curative therapy. Thus, there is an unmet clinical need for novel conditioning regimens that minimize toxicity without sacrificing therapeutic efficacy. Previously, we showed in mouse models that saporin-based antibody-drug conjugates (ADCs) targeting CD45 or cKit combined with Janus kinase 1/2 (JAK1/2) inhibitors enabled stable, high-level engraftment in fully mismatched HSCT with ≥99% donor myeloid lineage chimerism. However, saporin ADCs are not fully myeloablative, which limited their antitumor efficacy in prior studies and would likely limit the ability of these conditioning agents to provide protection against AML relapse. We hypothesized that alternative toxic payloads to saporin would enable generation of fully myeloablative ADCs capable of enhanced antileukemia benefit.
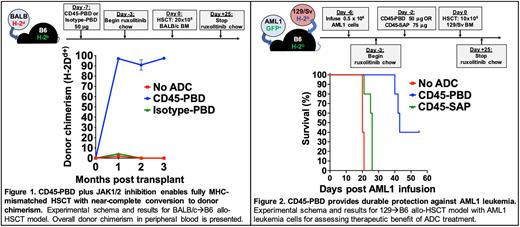
METHODS: We conjugated anti-mouse CD45.2 (clone 104), anti-mouse cKit (clone 2B8), and their respective isotype control antibodies with pyrrolobenzodiazepine (PBD) dimers via maleimide-thiol chemistry. PBD-conjugated ADCs were tested for cytotoxicity in vitro against the murine YAC-1 (CD45.2+) or P815 (cKit+) cell lines, as well as the human Jurkat (CD45.2-cKit-) cell line using XTT viability assays. Cytotoxicity against BM HSCs from B6 mice and AML1 primary leukemia cells (B6-derived, Dnmt3aR878H/+ and FLT3-ITD; provided by Dr. Timothy Ley) was tested using methylcellulose assays. HSC depletion in vivo was assessed 7 days after treatment with a single dose of CD45- or cKit-PBD. Syngeneic and fully mismatched HSCT studies with CD45-PBD were done by conditioning B6 mice with 50 µg ADC 7 days before transplant (d-7), then infusing 10x106 B6-GFP (syngeneic) or 20x106 BALB/c (allogeneic) BM cells on d0. For in vivo leukemia killing studies, B6 mice were infused with 5x105 AML1 cells on d-6, treated with 50 mg CD45-PBD or 75 mg CD45-saporin on d-2, then transplanted on d0 with 10x106 T cell depleted BM cells from 129/Sv mice. In allo-HSCT studies, mice received oral ruxolitinib (2 mg/g chow) starting on d-3.
RESULTS: CD45-PBD targeted murine YAC-1 cells and cKit-PBD targeted P815 cells in vitro with picomolar-range potencies; neither ADC was effective against human Jurkat cells. Target-specific killing was confirmed with blocking studies using excess unconjugated anti-CD45.2 or anti-cKit. While CD45-PBD and cKit-PBD showed similarly high efficacy and picomolar-range potencies at targeting AML1 leukemia cells in vitro, cKit-PBD was approximately 40-fold more potent at depleting B6 HSCs in vitro than CD45-PBD (IC50 values: 243 fM for cKit-PBD versus 10 pM for CD45-PBD). In vivo, a single 50 µg dose of CD45-PBD or 25 µg dose of cKit-PBD completely ablated the HSC niche in B6 mice. Initial transplantation studies done with CD45-PBD confirm that this ADC enables both syngeneic and allogeneic HSCT with near complete donor chimerism in all recipient mice (Fig. 1). Finally, a single CD45-PBD dose provided durable protection against AML1 leukemia, whereas the nonmyeloablative CD45-saporin was only minimally protective (Fig. 2).
CONCLUSIONS: We successfully generated fully myeloablative CD45- and cKit-ADCs using PBD as the toxic payload. Notably, CD45-PBD enabled HSCT with near total conversion to donor hematopoiesis and was highly protective against the aggressive AML1 primary leukemia. Studies thus far with cKit-PBD have shown superior killing of murine HSCs in vitro and in vivo compared to CD45-PBD; testing of cKit-PBD in HSCT models and in vivo AML1 leukemia killing studies are in progress. Given the high potency of the PBD payload, we are conducting extensive histopathologic studies and serum chemistry testing to evaluate the acute and chronic organ toxicities of conditioning with PBD-based conjugates.
Disclosures
DiPersio:Amphivena Therapeutics: Research Funding; NeoImmune Tech: Research Funding; Macrogenics: Research Funding; BioLineRx, Ltd.: Research Funding; CAR-T cell Product with Washington University and WUGEN: Patents & Royalties; VLA-4 Inhibitor with Washington University and Magenta Therapeutics: Patents & Royalties; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; RiverVest Venture Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Research Funding; WUGEN: Current equity holder in private company, Research Funding; Magenta Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal